EXTENSIVE testing for the SARS-CoV-2 virus that causes COVID-19 is beginning to ramp up. By the end of April, the country will be able to conduct 10,000 tests per day, according to Department of Health Undersecretary Maria Rosario Vergeire.
Is this the mass testing that many have been clamoring for?
In a way, yes. But infectious disease specialist Dr. Edsel Maurice Salvaña prefers to call it “massive testing”. As he explained in a Facebook post, “To clarify, the goal is to test everyone who needs a test[.] [T]his includes close contacts of known cases and some sampling in the community.”
He continued: “This is not mass testing but massive testing to test everyone who needs it. Testing people who don't need the test is a waste and can lead to misleading data, given the limitations of testing accuracy.”
With more wide-scale testing rolling out, here’s our explainer on how, exactly, COVID-19 tests work. Hopefully, it will clear up a lot of questions and terms floating around now that widespread testing is being rolled out.
Warning: science words ahead!

First, let’s get some terms clear.
Viruses and diseases often have different names. For example, AIDS (acquired immunodeficiency syndrome) is the disease. The virus that causes it? HIV.
The respiratory disease that is currently raging around the world is called COVID-19. The virus that causes it has been named SARS-CoV-2. It is part of a family of viruses called the coronavirus, which include viruses that cause SARS and MERS. Early on in the start of its worldwide spread, SARS-CoV-2 was called the novel coronavirus (because it was a new form of coronavirus) or nCoV for short.
In this article, we’ll occasionally use the words coronavirus or novel coronavirus to refer to SARS-CoV-2.
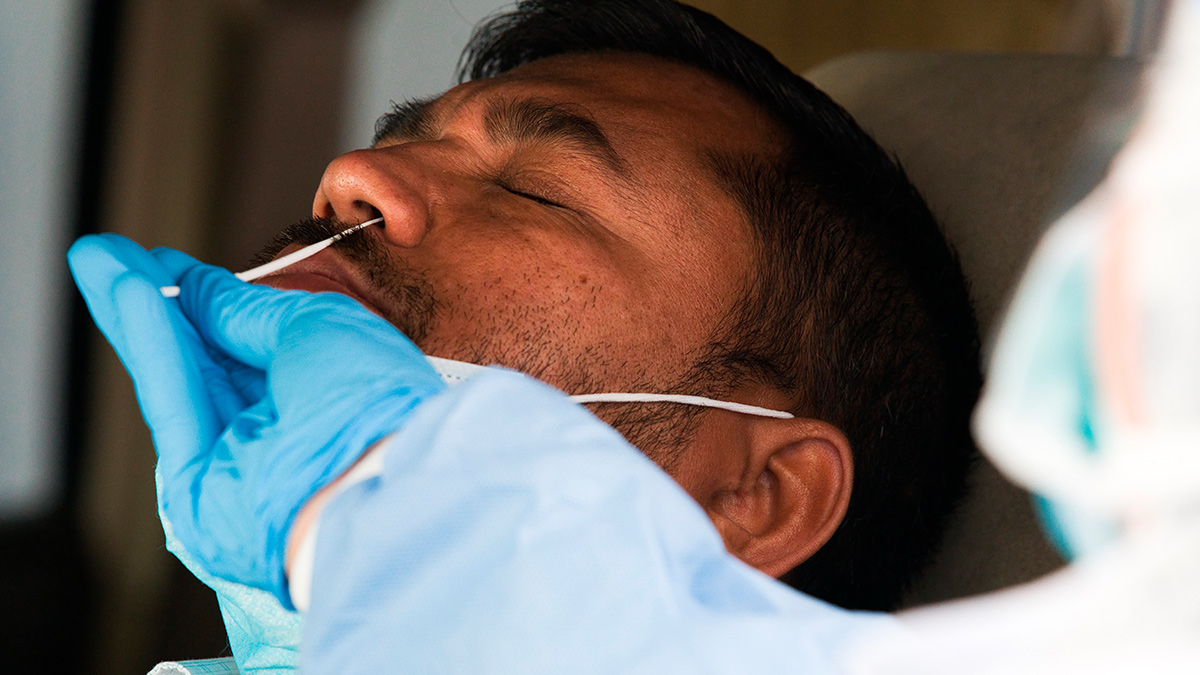
So how do we test for SARS-CoV-2?
In the battle against COVID-19, there are two kinds of tests that are being deployed.
One looks for the genetic material or protein evidence of the actual virus. The other kind of test looks for antibodies in your system that are fighting against SARS-CoV-2.
Let’s first talk about the first kind of tests.
The main methodology for checking for genetic material of the virus is the polymerase chain reaction, or PCR, test. A quantitative reverse transcriptase-based PCR test (or RT-PCR, for short) remains the “gold standard in determining whether a person is infected or infectious,” according to a DOH circular dated March 31.
As of April 3, the Philippine Food and Drug Administration has approved 21 RT-PCR tests for use in the country — including the one locally developed by the University of the Philippines and manufactured by Manila Healthtek Inc.
How does RT-PCR work?
Basically, RT-PCR tests look for genetic evidence of the virus in a given sample. A patient is swabbed in the nose and throat (since COVID-19 is a respiratory disease), and this swab is brought to a central testing laboratory.
Because the novel coronavirus is made up of a single strand of genetic material called RNA, it needs to be converted into double-stranded DNA so that it can be “copied” for further analysis. Scientists use a well-known process called reverse transcription to do this. So they extract RNA from a sample, then use an enzyme to reverse-transcribe it to DNA.
The RT-PCR machine then essentially acts like a genetic xerox machine, churning out billions of copies of the DNA sample. The “amount” of the virus is then measured using a fluorescent dye. If the fluorescence goes above a certain level, then it’s a hit, and the presence of the virus is confirmed in the sample.
What are RT-PCR’s advantages?
They are highly sensitive, highly accurate (especially if administered early, and they are very easy to manufacture. On April 3, the secretary of the Department of Science and Technology announced that Manila Healthtek Inc., manufacturer of the UP-developed test, had already received reagents that would enable them to make up to 120,000 kits.
What are RT-PCR’s disadvantages?
Results often depend on the quality of the sample. “As the swab needs to pick up enough of the virus to work, this is one of the reasons the coronavirus in the PCR test can have a higher false negative test rate — that is, missing the virus even though someone has the infection,” explained Dr. James Gill of Warwick Medical School in the UK.
This is why repeat RT-PCR tests may need to be conducted on a single patient.
And while the actual testing process is very fast, samples need to be transported to a central testing laboratory. For the first few weeks of ECQ, there was only one in the country: the Research Institute of Tropical Medicine in Muntinlupa. There are now, however, six, with more already in the last phase of accreditation.
Still, the need for a centralized point of analysis means turnaround times for results could be slow.
And what about the second kind of tests, the antibody tests?
In these kinds of tests, you’re not looking for the virus itself, but for evidence that the virus is (or was) inside your body. That means looking for antibodies — proteins manufactured in your blood that act as virus fighters.
Currently, the FDA has approved nine antibody test kits for use in the Philippines.
Some of these tests are laboratory based. But of particular interest to many people are the rapid antibody test kits, which are portable, don't need a specialized laboratory, and, as their name suggests, gives fast results.
What antibodies is this test looking for?
The antibody known as immunoglobulin M, or IgM, shows up anywhere from 5 to 10 days from when you first get infected. Then, there is immunoglobulin G, or IgG, which appears within 10 to 14 days.
“Many tests can distinguish between IgM and IgG and thus give information on the phase of infection (early/current versus later stage/previous infection),” said Dr. Andrew Preston, a microbial pathogenesis expert from the University of Bath.
So how does the test work?
Developing an antibody test is a little tricky. To make a RT-PCR test, you only needed to know the full genome sequence of SARS-CoV-2, which China provided back when the coronavirus began to explode. But to make an antibody test, you had to study the protein coat around the virus — specifically, the proteins that would trigger an antibody response.
Once you’ve identified the proteins in the viral coat, you have to produce them in a laboratory and embed them into a matrix called an immunoassay. “[T]heir development takes time. Expressing the protein in the right structure is often the most difficult step,” wrote Anna Petherick for The Lancet.
However, once manufactured, rapid diagnostic antibody tests are portable, rapid, and can be used in point-of-care (POC) situations. For example, a lateral flow immunoassay test — “essentially a dipstick encased in a cassette,” as Nature described it — only needs two drops of blood. Results appear in less than an hour.
Sounds handy, right? However, in its guidelines on COVID-19 test kits, the DOH says that only medical doctors can prescribe rapid antibody test kits, as well as interpret the results. It also cannot be bought over the counter.
What’s the disadvantage of the antibody test?
Sure, rapid diagnostic antibody tests are fast and portable. But, as we described above, they’re also quite difficult to test and develop.
You also have to remember that antibodies don’t appear in your blood right away. So it’s very important to know when to take the test. Take it too early, when your body hasn’t developed any antibodies yet, and you might get a false negative result. Because of this, they are regarded as less accurate than RT-PCR tests.
In the interim guidelines on COVID-19 testing by the Philippine Society for Microbiology and Infectious Diseases, the organization wrote: "The true accuracy of immunoassays for COVID-19 has not been established yet. The likelihood of false negative results from immunoassays in general should be considered. Furthermore, analytical specificity and sensitivity have not yet been determined."
Can rapid antibody tests be used to mass test for COVID-19?
The DOH makes its rules clear in its March 30 memo: “All rapid antibody-based test kits cannot be used as a standalone test to definitively diagnose COVID-19.” In addition, all reports from the department will be based only on RT-PCR test results, and “no public funds shall be used to pay for any COVID-19 rapid antibody-based test kit.”
In the Philippines, for now, rapid antibody test kits can only be used by private medical institutions as an “adjunct tool” and a supplement for RT-PCR tests. For example, they can be used to clear patients who do not exhibit symptoms and have completed a 14-day quarantine after being released from a health facility.
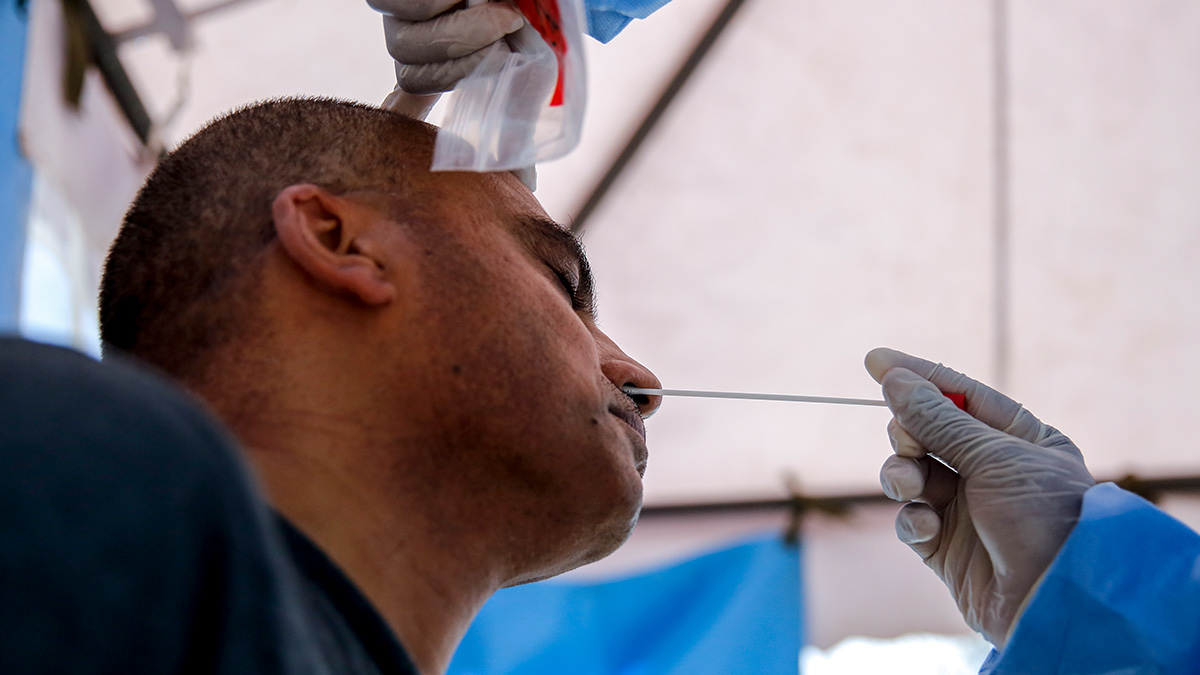
Is there another kind of test?
There is. It’s called an antigen test. It’s similar to the RT-PCR test because it looks for evidence of the virus itself, and not evidence that your body is fighting it. But if an RT-PCR test looks for a virus’ genetic material, an antigen test looks for the specific proteins (called antigens) found in the outer coats of SARS-CoV-2. Various biotech companies are working on making small, portable versions of these, similar to rapid antibody tests. However, these are still in development, and the Philippine FDA has not authorized any for use.
Quotes from UK experts from Science Media Centre.
Source: Spin PH
0 comments: